We are full-service, at full speed, fully on the pulse of innovation.
With decades of disposable medical device and sterile packaging manufacturing experience, we know if a great idea isn’t developed with pace, or manufactured with precision, key market milestones will be missed.
Our customer-first design and development approach is uniquely customized to each client and solution. Whether you need a partner for one phase of development, or all phases from design and development through manufacturing, our team of experts can guide you every step of the way.
-
-
-
-
-
-
-
-
-
Why Choose
Hover to learn more Tap to learn more
UFP MedTech?
Infection Control
Protective Packaging
Surfaces & Support
Interventional & Surgical
Therapeutics
Wearables
Wound Care
Diagnostics

Forging A Path Forward
Much like our focus on shaping design and engineering innovation, UFP MedTech was forged through decades of continuous advancement. Discover the milestones that made us who we are today.







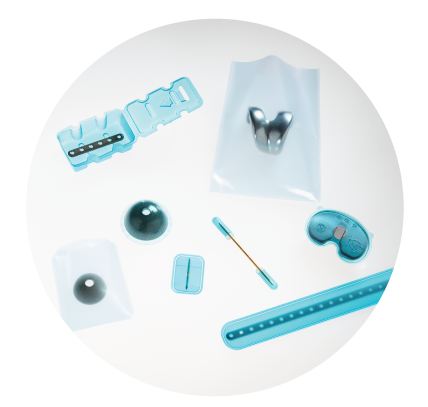














Passion. Pride. Precision. People.
When you partner with UFP MedTech, you get unlimited access to leading experts: medical device design, development, engineering, production and material specialists—each one, an extension of your team. Our core values of responsiveness and leadership drive us to better serve you and create superior outcomes with enhanced speed to market.

Chicopee, MA

Newburyport, MA

Georgetown, MA

Grand Rapids, MI

Chicopee, MA

Grand Rapids, MI

Grand Rapids, MI

Chicopee, MA

Request a Consultation