Markets
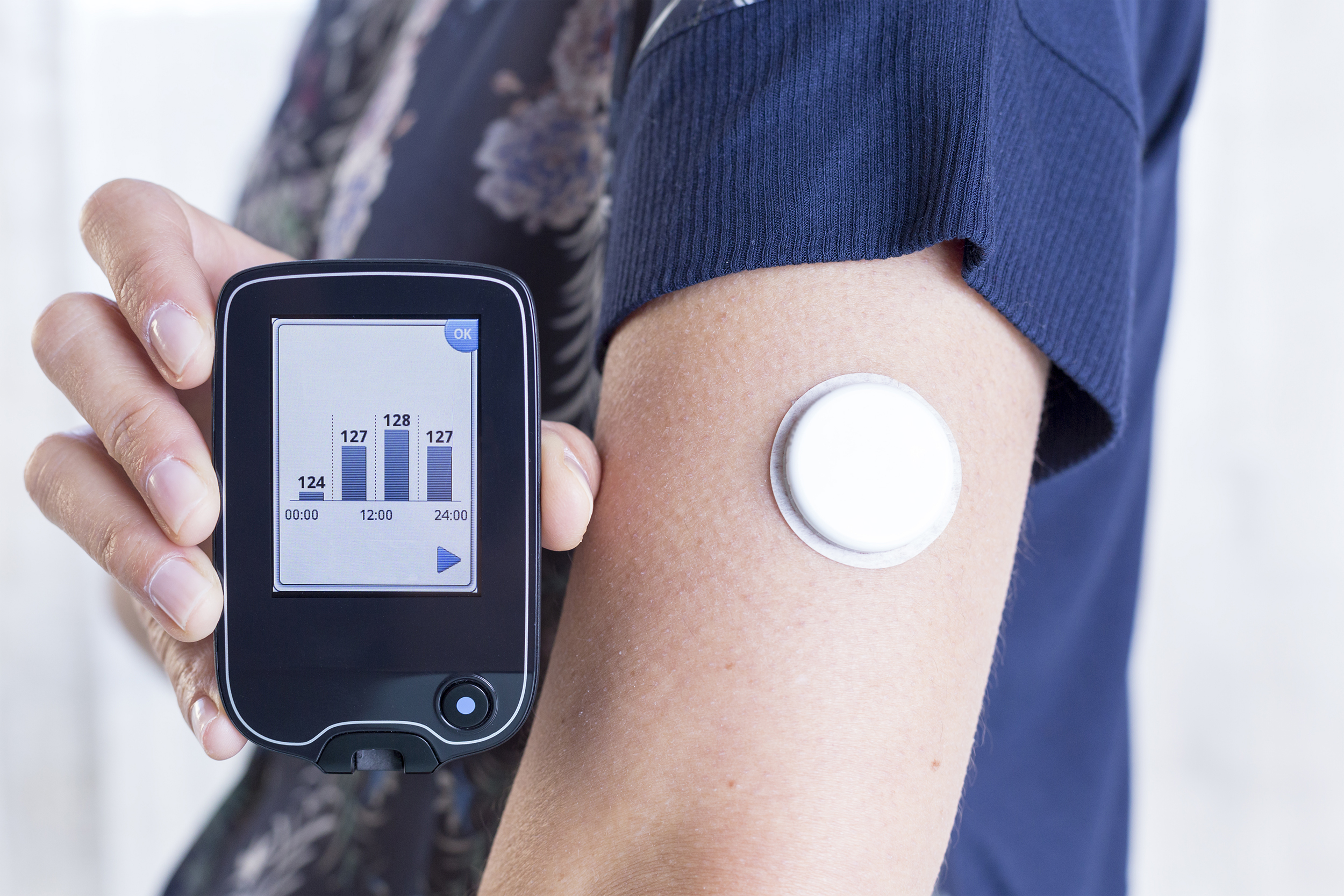
Medical Wearables
In the world of medical wearables, the right enclosure system is crucial for optimal performance. Leveraging our expertise with foams, films, adhesives, and fabrics, we help create solutions that not only safeguard the device but also provide patient comfort and aesthetic appeal.
Specializing in medical-grade alternatives to silicones, our portfolio features closed-cell foams, adhesives, thermoplastic films, and fabrics. We select the optimal materials based on your device’s size, usage duration, body location, and how it will be disposed, to provide solutions that offer:
- Secure Mechanical Bonds: Our advanced thermoplastic welding techniques create superior bonds that surpass the limitations of traditional adhesive systems.
- Patient Comfort: Designed for wearability and ease of use.
- Aesthetic Appeal: Blending functionality with sleek, appealing designs.

Applications
- Diagnostic Patches and Biosensors
- Insulin Patches
- Exoskeleton Components
- Transdermal Delivery Systems
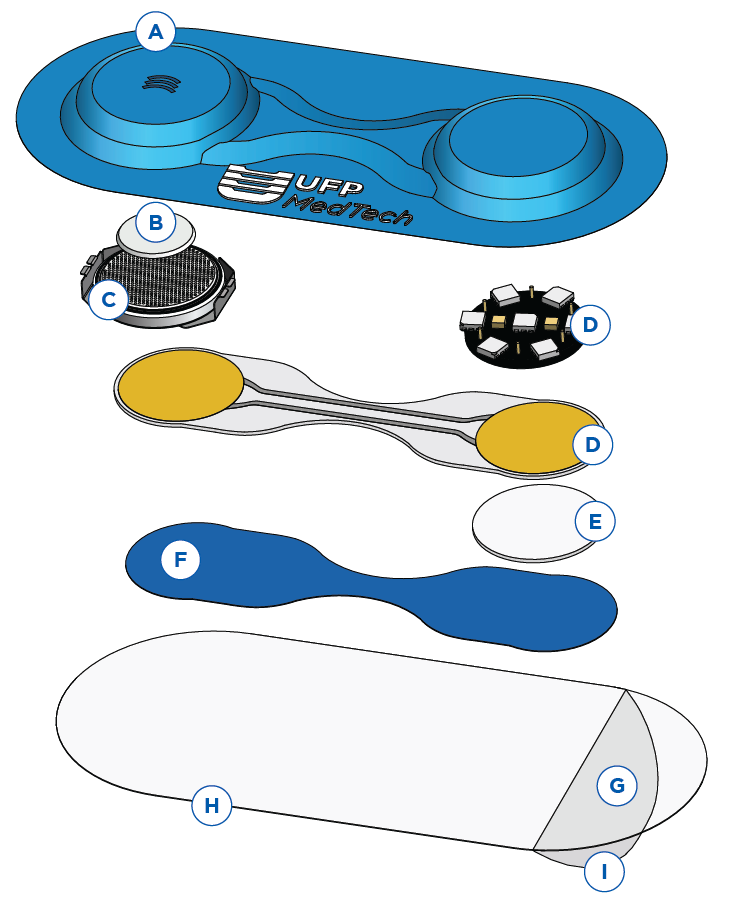
Exploring the Components:
(A) Thermoformed Exterior Cover
Formed exterior shell that provides a sleek, low-profile, flexible cover for your device that safeguards the device’s components without compromising functionality or aesthetics.
(B) PTFE Filter
Enables airflow for the power source while blocking water and steam from entering the device.
(C) Power Source
A compact battery or power cell.
(D) Customers’ IP
Customer owned technology, electronics, or other IP placed within the formed exterior cover.
(E) Hydrogel Sensor
Hydrogel-based sensor to monitor vital health indicators.
(F) Insulation
Designed to encompass the device’s internals, this insulation enhances efficiency through effective thermal management.
(G) Pressure Sensitive Adhesive
A carefully selected, skin-friendly adhesive ensuring strong, biocompatible adherence to the body, suitable for use from 3 to 24 days.
(H) Secure Bond
Advanced thermoplastic welding techniques create impermeable seals.
(I) Release Liner
Custom release liner designed for easy application onto the user.
To request a consultation, contact us below or give us a call at 866-921-4718
* denotes mandatory fields.

