At a time when patients use ambulatory surgical centers (ASCs) for same-day procedures more than ever, these facilities need to be prepared for an even greater demand. ASCs are expected to see:
- 12% growth in the next 5 years1
- 22% growth in the next 10 years1
- 40%–60% of all joint replacements in the next 5 years2
The shift of joint replacement and other orthopedic cases from hospital settings to ASC centers —which accelerated during the pandemic—is especially impactful. These trends will only continue to grow, and OEMs must evolve product portfolios to help ASCs meet the demand by moving toward sterile-packaged implants and single-use devices.
3 Key Challenges for ASCs
ASC facilities survive, and thrive, on a high case volume to overcome their typically small operating budgets. In light of the ongoing increase in same-day surgeries and outpatient procedures, ASCs face three significant hurdles compared to hospitals:
- Lower reimbursement
- Limited physical space
- Insufficient staffing
All of which puts pressure on OEMs to develop ASC-specific solutions that cost less and take up less space—without creating more work for healthcare providers.
3 Big Opportunities for OEMs
OEMs have an opportunity to recalibrate their offerings and invest in solutions that can:
- Enable ASCs to boost case volume and drive more revenue
- Empower surgeons to improve efficiency and safety
- Enhance patient recovery while reducing cost of care
By focusing on the unique needs of ASCs—and partnering with a company like UFP MedTech—OEMs can maximize success.
3 Proven Solutions from UFP MedTech
UFP is prepared to help OEMs with this burgeoning demand for cost-effective, easy-to-use solutions that are smaller, single-use, and ready right out of the box.
1. Packaging Components
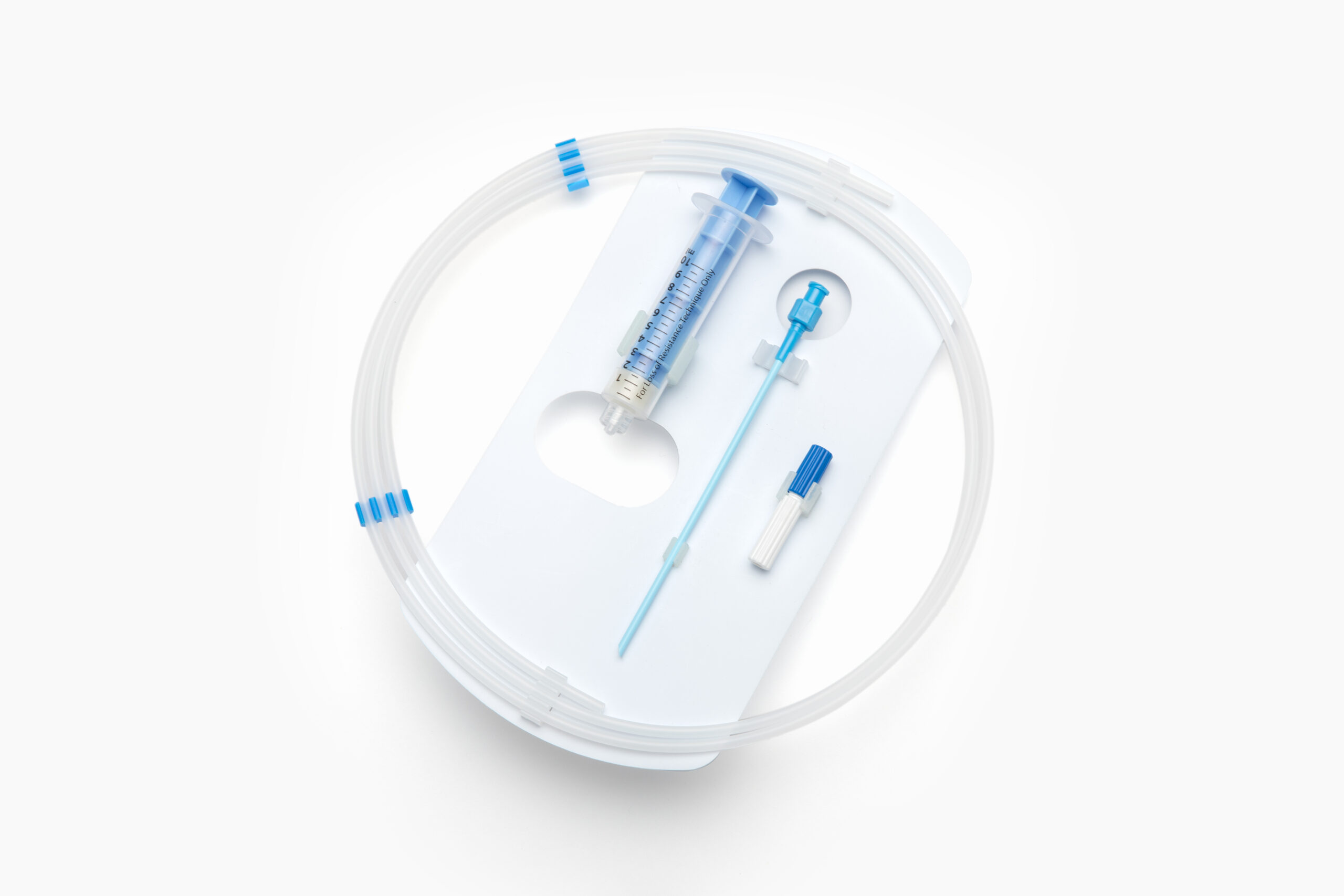
Protective and sterile packaging solutions can be developed to cater to specific needs such as providing visibility of the product within the packaging, having embossed part numbers, or ensuring user-friendly aseptic presentation from the sterile barrier system. They offer sterile barrier protection, provide puncture and abrasion resistance, and for ASC’s must be:
- Easy to use – Less training needed to open and deploy
- Space saving –Smaller components that store flat
- Fully sterilizable – Pre-sterilized solutions for ASCs
- Easy to dispose – Less bulky than thermoformed trays
Explore EZ Card®, FlexShield® & Dispenser Coils
2. Surgical Robotic Drapes

Single-use surgical robotic draping systems and equipment covers preserve the sterile barrier while allowing for a full range of motion. They combine puncture resistance with exceptional flexibility, and for ASC’s must be:
- Easy to use – Efficiently deploy, install, remove, and dispose
- Space saving – Carefully designed to ship and store flat
- Fully sterilizable – Pre-sterilized solutions for ASCs
- Easy to dispose – Smaller environmental footprint for less waste
Explore Surgical Robotic Drape Systems
3. Minimally Invasive Surgical Devices

Single-use devices and components for interventional and surgical procedures play a key role in fewer complications, faster recovery, and less pain for patients. UFP custom-develops an array of minimally invasive surgical devices, components, and packaging—incorporating materials like films, foams, plastics, and composites – that are ideal for ASC outpatient procedures.
Explore Minimally Invasive Surgical Devices. For turnkey solutions, partner with UFP to design bespoke, single-use procedure kits that provide an all-in-one package for specific surgical needs.
Learn more about UFP MedTech’s ASC solutions now or contact our team to get started.
References:
1 Sg2 2023 Annual Report Forecasts Significant Growth in ASC Volume
2 Zimmer Biomet’s Ivan Tornos sets priorities for first year as CEO


