Creating a successful medical device or single-use product is only half of the battle when it comes to manufacturing and mass-producing your solution. How will it be packaged and sterilized according to medical-grade standards and regulations?
First, selecting the right materials for packaging is critical and complex, with varied implications to the functionality and cost of each product. In fact, due to its protective nature, functional, and sterile qualities, packaging is really part of the product itself. These are the key criteria and questions to ask when discerning which materials would work best for your unique solution:
- Functionality: Will the packaging material allow for the device to be properly opened and used without error?
- Physicality: Will the packaging design stand up to shipping, use materials that are right-sized, and maintain product sterility?
- Chemical Elements: If any bonding or binding processes are used to contain the product within the package, will there be any fluid compatibility issues?
- Electrical Components: Will the packaging material’s conductivity or dielectric strength affect the device?
- Thermal Components: What is the melting point and thermal conductivity of the product and packaging materials for withstanding extreme temperatures through transit and sterilization?
- Cost: How will the selected materials affect the minimum order quantity (MOQ), price per product (PPP) and overall timeline of cost to manufacture?
Perhaps one of the most important questions to ask is: will the product and packaging materials sustain the sterilization methods required to make the product sterile?
Below is an outline of the most popular sterilization methods and how they compare.
Sterilization Methods
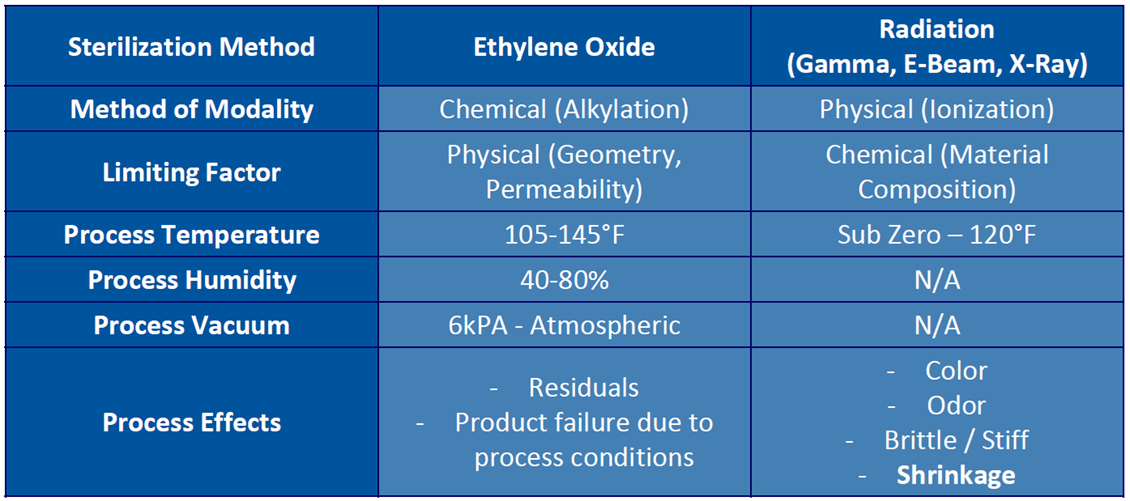
Ethylene Oxide (EO) – Derived from plants and cooking oils. Lethality is the result of Alkylation (Replacement of hydrogen atom with an alkyl group).
- EO sterilized materials are not exposed to damage from excessive heat, moisture, or radiation
- Devices already packaged for shipment can be sterilized
- Significantly longer process times than radiation
Radiation
- E-Beam – Utilizes high energy electrons as its form of radiation. Electron bombardment breaks into the DNA of microorganisms.
- Provides the fastest dose delivery as compared to other radiation methods – seconds compared to minutes, hours, or day(s) for EO sterilization
- Can deliver a range of doses, from very high to very low
- Beneficial when product has a biologic component (tissues)
- When to use
- Low-Medium bulk packed density
- Product packaging / orientation is consistent
- Product consists of lower density plastics
- Product has a biologic component (tissues)
- Product degrades when processed via Gamma or X-Ray
- Gamma – Utilizes radioactive Cobalt 60 to emit photons to kill microorganisms.
- Compatible with most materials
- Materials “APT” to fail are Acetals, Propylenes (Unstabilized), and Teflons
- Offers good penetration of dense products and packouts
- Whole pallet can be processed at once, though pallets are commonly broken down due to the existing gamma sterilization systems
- Certain materials can become discolored or brittle
- Compatible with most materials
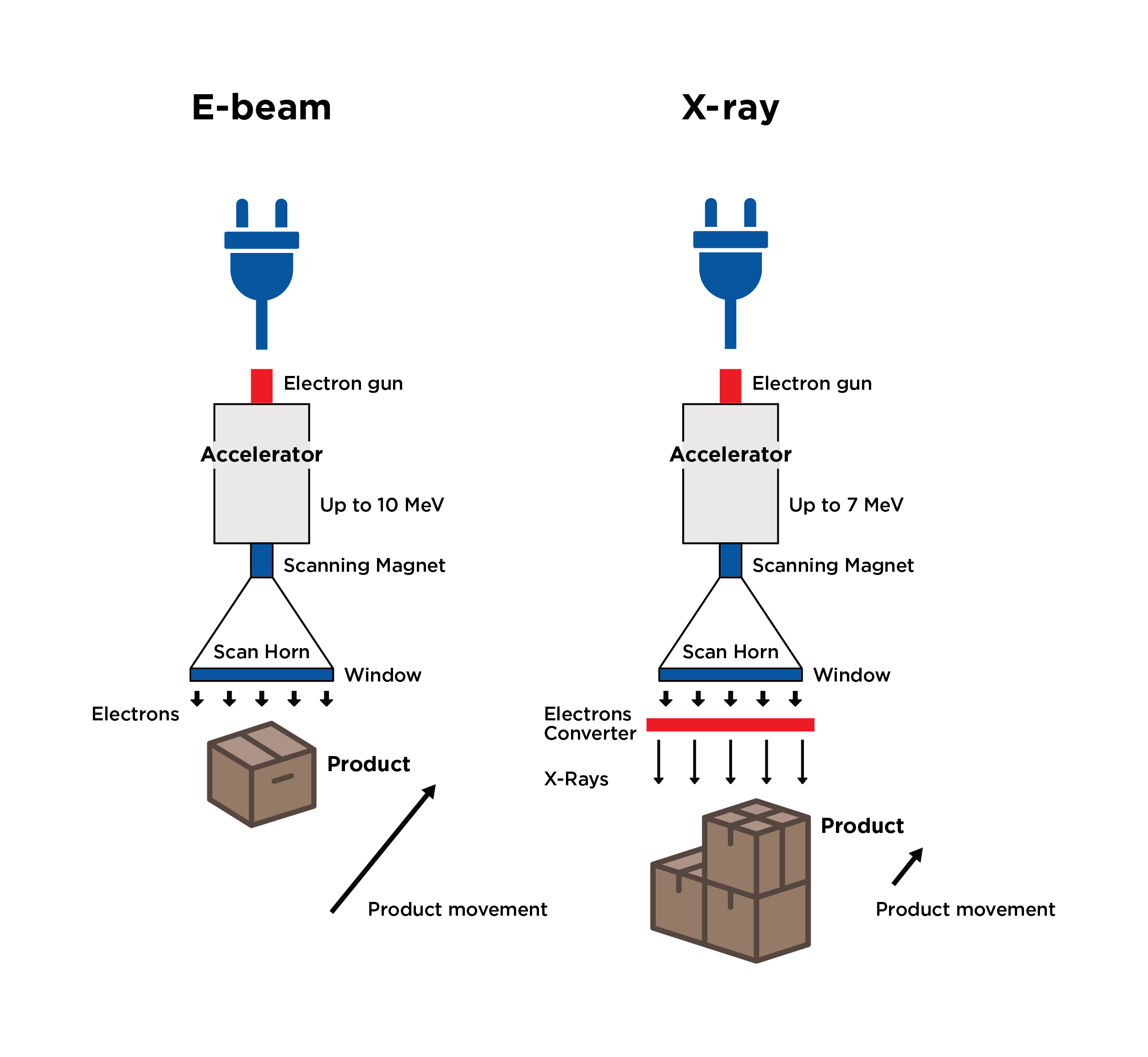
- X-Ray – Utilizes photon radiation, like gamma. X-ray starts as an electron beam with radiation being produced through bombardment and deceleration of particles. A tantalum plate is used at the end of the electron beam’s scan horn to capture electrons while allowing photons to penetrate through the packaging to sterilize the medical device.
- Similar benefits to Gamma
- Compatible with most materials
- Offers good penetration of dense products
- Short exposure time compared to gamma (minutes vs hours)
Comparison of Sterilization Methods
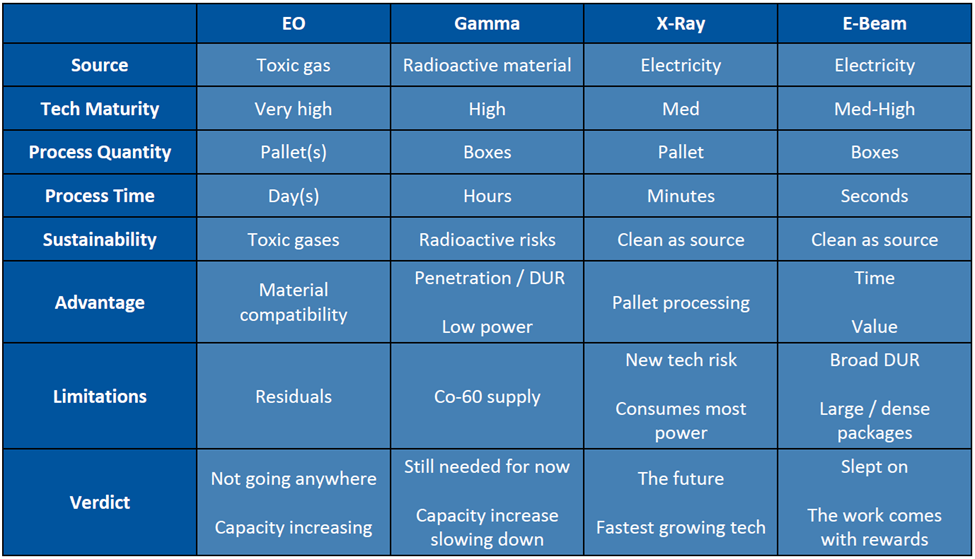 *DUR = Dose Uniformity Ratio (ratio of maximum dose allowed to minimum dose required)
*DUR = Dose Uniformity Ratio (ratio of maximum dose allowed to minimum dose required)
Bioburden
- Before choosing a sterilization method, it needs to be understood what microbes are present on the product
- Certain sterilization methods are more effective on particular microbes than others
Testing
- Sterilization validation is important since it proves that the product can be sterilized.
- ISO 11607-1:2019 covers packaging for terminally sterilized medical devices
- Additionally, testing should be performed during the development process as well as throughout the life of the product.
- This includes aging testing to determine packaging lifespan
There are other sterilization methods not covered here such as Vaporized Hydrogen Peroxide and Steam, but they are more commonly used for sterilizing instruments or devices on site (at the hospital for example) and not necessarily packaged devices.
Overall, it is crucial to select packaging materials that are compatible with the chosen method or methods of sterilization. Remember, packaging is a key component of the overall device and performs important functions such as containment, protection, instructions for use, and product identification. Packaging must maintain its sterile barrier, while performing its primary duties throughout the supply chain, up through its point of use.
To learn more about these sterilization methods, and how they may affect your medical device or product, reach out to our packaging team at UFP MedTech.


