Problem:
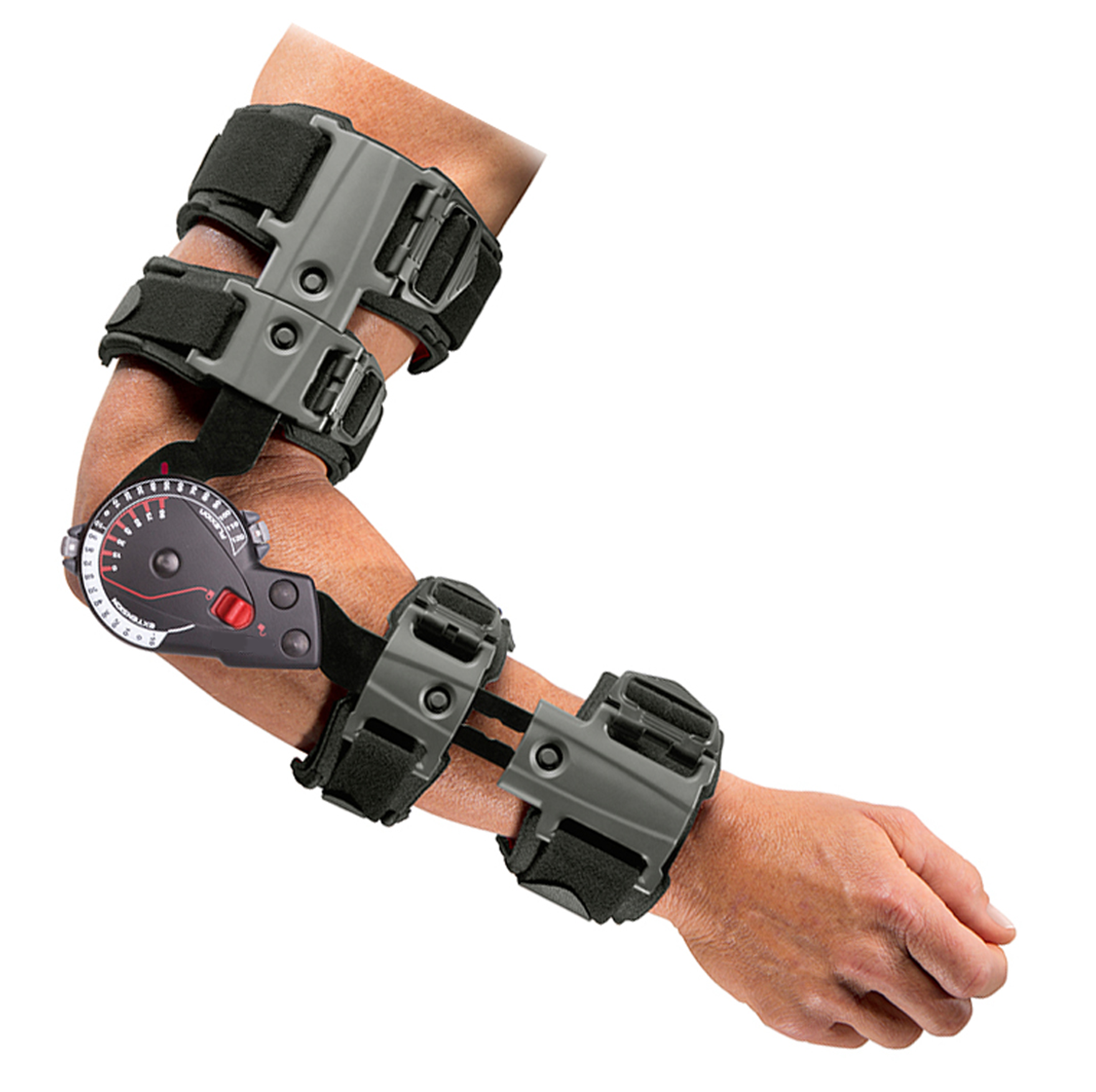
One of the largest global orthopedic bracing corporations discovered they had fallen behind their competition for upper extremity braces. Specifically, a brace for the arm.
The new braces they developed include soft structural components that provide comfort and protection to the patient during use. In most cases these components are made from fabrics and aluminum, making them pliable to fit patients of various sizes. At the time, their current supplier of the soft components was unable to solve a reoccurring problem of the delamination of the fabric to the aluminum, a common issue in extremity bracing. The edges were becoming exposed and putting the patient at risk of being cut. The orthopedic bracing company approached UFP MedTech with their dilemma. We immediately got into action, utilizing our engineering and material expertise.
Solution:
Through innovation, resourcefulness and design, UFP MedTech was able to custom engineer and manufacturer a combination of components incorporating foams and fabrics that met the customer’s high demands. Utilizing our proprietary compression molding and heat sealing fabrication capabilities, we created a solution to ensure the metal would not tear through the edges of the pad.
Customer Success:
Our customer’s issues with delamination were eliminated. Their brace provided the longevity their customers desired, without the worry of component failure and exposing the sharp aluminum edges. The bracing company was then able to incorporate the foam and fabric technologies into a new, first-to-market product in their highly competitive industry.
Learn more about UFP MedTech’s therapeutic solutions.